Explain Difference Between Miscible and Immiscible Liquids
1 Miscible Liquid can be easily dissolved in any other liquids but Immiscible Liquid do not dissolve in other. The less dense fluid will rise to the top.

3 Miscible And Immiscible Liquids Ppt 25 November 2015 Today S Title Miscible Or Immiscible Aims Define The Terms Immiscible And Miscible F Course Hero
You then close the tap before the other liquid starts to run out.
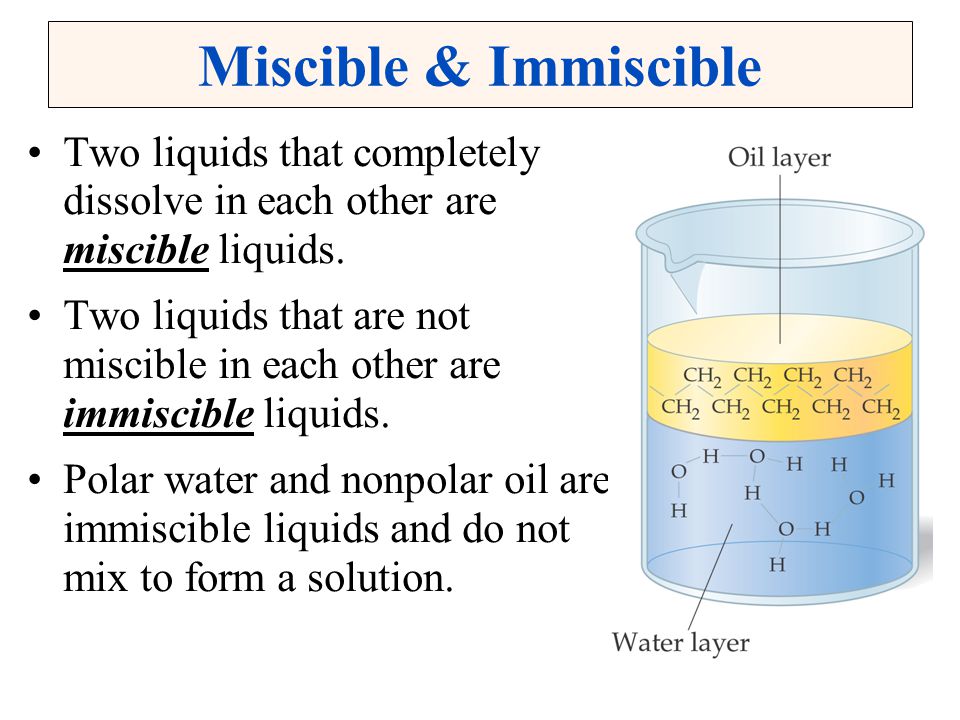
. The upper liquid can then be put into another beaker. As for example a type of immiscible liquid is oil and water. A distinct layer between the two liquids will not form when we have a solution which is miscible.
Is acetic acid and water miscible. For example a type of immiscible liquid is oil and water. Immiscible liquids are those liquids that dont mix to give a single phase.
Thus honey and water are miscible liquids. Are miscible liquids while Oil in water is immiscible. Liquids are said to be miscible with each other because the will mix to form a homogeneous solution.
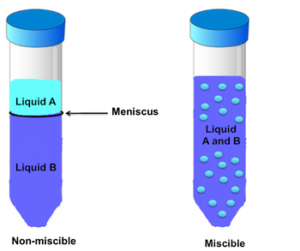
The more dense component will sink. Miscible liquids are mutually soluble meaning they can be mixed together to form stable solutions. Thus when a distinct layer does form in a mixed solution this is called immiscibility.
When water and organic acids are mixed for example two layers remain with the water layer containing some acid and the acid layer including some water. Allow the lower liquid to run out the funnel and be collected in a beaker while the tap is open. Immiscible liquids will dissolve into one another to some extent but there will be mixing ratios that cause a bilayer.
Components of an immiscible mixture will separate from each other. What are immiscible liquids. A miscible liquid can be dissolved readily in some other liquid.
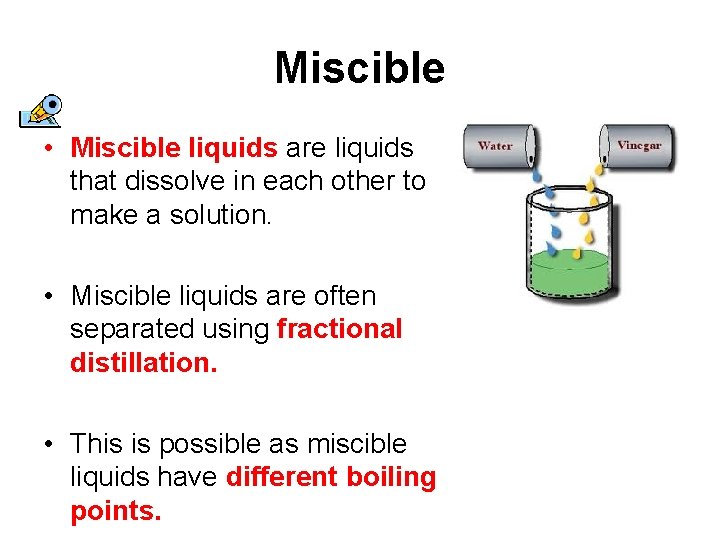
4 rows Miscible liquids. A miscible solution is any solution that can be mixed together to form one liquid phase. Honey is soluble in water.
Miscible liquids are liquids that can mix well with one another to form a homogenous solution. When this occurs they are often referred to as partially miscible. Explain the difference between oxidation and reduction.
Immiscibility refers to the inability of two substances to combine to produce a homogenous mixture. Miscibility refers to the ability of one liquid to completely dissolve in another liquid solvent. Miscible liquids are liquids that can mix well with one another to form a homogenous solution.
Answer Immiscible is the property where two substances are not capable of combining to form a homogeneous mixture. Immiscible liquids are not capable of being mixed together to form stable solutions. Many liquids fall somewhere in between miscible and immiscible.
Im going to assume you mean immiscible and miscible liquids. The velocity field u of such incompressible fluids is not in general solenoidal divu0. Immiscible solutions do not mix together and instead form separate layers.
This is possible as miscible liquids have different boiling points. Examples of solutions created with miscible liquids include. When a distinct layer does form in a mixed solution this is called immiscibility.
As adjectives the difference between insoluble and immiscible is that insoluble is that cannot be dissolved while immiscible is physics of two or more liquids. 2 If the liquid is immiscible you will be able to see a meniscus on the liquid while for miscible liquids you will not. Incompressible miscible liquids with gradient stresses.
Gasoline is a mixture of many organic solvents such as benzene toluene xylenes and others. Can be separated using separating funnels. What is the difference between miscible and immiscible liquids.
In miscible liquids there will be no creation of a layer between the two liquids. A distinct layer between two liquids will not form when you have a solution that is labeled miscible. A distinct layer between two liquids will not form when you have a solution that is labeled miscible.
Miscible liquids are often separated using fractional distillation. The components are said to be Immiscible. When a distinct layer does form in a mixed solution this is called immiscibility.
The immiscible liquid does not dissolve in other liquids. A conservation form of the left hand side of the diffusion equation which differs from the usual. Oil and water-oil float on top of the water honey and oil.
4 rows The two liquids that dissolve in each other in all proportions are called miscible liquids. The density of incompressible fluids can vary with concentration φ and temperature but not with pressure. Alcohol and water milk and water.
What is difference between miscible and immiscible liquids. Is Honey miscible or immiscible. Are liquids that do not form a solution but separate into two layers.
Alcoholic drinks contain ethanol and water. For instance ethyl acetate will dissolve in water up to 83 g per 100 mL of solution. Can observe meniscus on the liquid.
After that it forms a bilayer similar to a vegetable oil-water mix. Miscible Miscible liquids are liquids that dissolve in each other to make a solution. Liquids which mix together in all proportions and.

Write The Differences Between Miscible And Immiscible Liquids How Can You Separate Them Brainly In
Explain With Suitable Diagram Two Different Processes Used For Separating A Mixture Of Two Liquids Both Miscible And Immiscible Chemistry Topperlearning Com Epdv9jnn

Miscible Liquids Definition Examples Video Lesson Transcript Study Com

Today S Title Miscible Or Immiscible Ppt Download
How Can We Separate A Mixture Of Two Miscible Liquids A Plus Topper
What Examples Of Miscible Liquids Are There Quora

Miscible Liquids Definition Examples Video Lesson Transcript Study Com

Miscible Or Immiscible Ppt Video Online Download

What Are Miscible And Imiscible Liquids Brainly In

8 Miscible Liquids Examples In Daily Life Studiousguy

Miscible Liquids Definition Examples Video Lesson Transcript Study Com
Illustrated Glossary Of Organic Chemistry Miscible Immiscible

Miscible Or Immiscible Ppt Video Online Download

Difference Between Miscible And Immiscible Liquids Compare The Difference Between Similar Terms
Solutions Ppt Video Online Download
25 November 2015 Todays Title Miscible Or Immiscible

Miscible Definition In Chemistry What Is Miscibility

3 Miscible And Immiscible Liquids Pdf Liquids Distillation

Difference Between Miscible And Immiscible Liquids Compare The Difference Between Similar Terms
Comments
Post a Comment